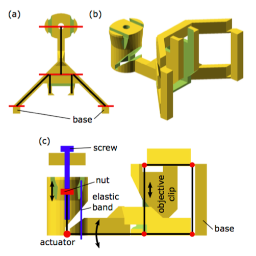
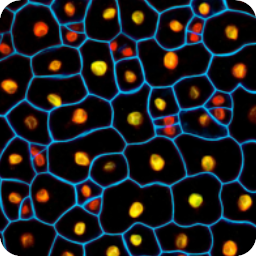
Plant and microbial microscopy and visualisation
The last twenty years have seen a revolution in the application of optical techniques to the study of biological systems. This largely due to the development of highly specific fluorescent labelling methods, and optical techniques such as confocal laser scanning microscopy.

Below are inks to a collection of confocal microscopy images from the Haseloff lab - including fluorescent protein labelled transgenic plants, stained wholemounts and 3D reconstruction of plant cell anatomy. Images collected by John Runions, Elisabeth Truernit, Laurent Laplaze, Fernan Federici, Sarah Hodge, Smita Kurup and Jim Haseloff.
Techniques:
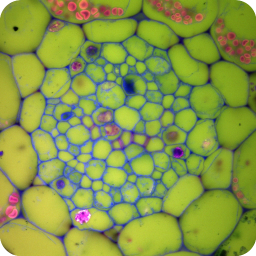
Confocal microscopy images of fixed plant anatomical specimens in the Department of Plant Sciences, University of Cambridge.
Images collected by Jim Haseloff
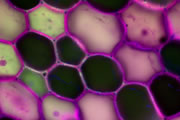
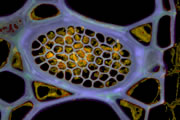
A wide variety of staining techniques have been adopted for plant specimens over the past 150 years. Perhaps the most widely used general tissue stains are Safranin O and haematoxylin. These are often accompanied by the use of counterstains such as Fast Green, Orange G, or Alcian Blue.
In addition, there is a large variety of more specific staining techniques that have been developed for particular plant materials and organelles. For example, Feulgen staining has been used for the specific labelling of DNA, the periodic acid-Schiff reaction can be used to label carbohydrates, Aniline Blue for callose, Nile Red for oil bodies, and Phloroglucinol for lignin. A multitude of published protocols are available. An excellent published source of procedures can be found in Plant Microtechnique and Microscopy by Steve Ruzin, Oxford University Press, Oxford, UK. 1999.
Interestingly, many of the synthetic dyes used for plant microtechnique are highly fluorescent. This is particularly true for red, orange, and yellow dyes in the azine (e.g., Safranin O), acridine (e.g., Acridine Orange), and xanthene (e.g., Rhodamine) families. Thus, many classical histological techniques unintentionally produce specimens that are intensely fluorescent. In addition, aldehyde fixation, certain mountants, and long-term storage of stained preparations can result in tissue fluorescence, and the high concentration of stains deposited in the sections can lead to metachromasia. In our hands, it is rare to find stained and sectioned botanical material that is not highly fluorescent.
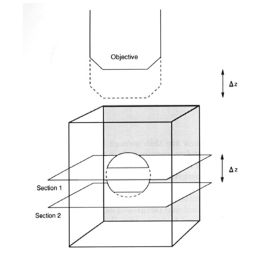
The digital controls of a confocal microscope allow for the clean separation of different fluorescent emission signals and the balancing of signal levels in different channels. Thus, fluorescent images of exceptional clarity and vivid color can be easily obtained. In addition, the optical sectioning properties of the confocal microscope can be used to collect clear images from within thick sections and wholemounts.
Images collected by Jim Haseloff
A wide variety of staining techniques have been adopted for plant specimens over the past 150 years. Perhaps the most widely used general tissue stains are Safranin O and haematoxylin. These are often accompanied by the use of counterstains such as Fast Green, Orange G, or Alcian Blue.
In addition, there is a large variety of more specific staining techniques that have been developed for particular plant materials and organelles. For example, Feulgen staining has been used for the specific labelling of DNA, the periodic acid-Schiff reaction can be used to label carbohydrates, Aniline Blue for callose, Nile Red for oil bodies, and Phloroglucinol for lignin. A multitude of published protocols are available. An excellent published source of procedures can be found in Plant Microtechnique and Microscopy by Steve Ruzin, Oxford University Press, Oxford, UK. 1999.
Interestingly, many of the synthetic dyes used for plant microtechnique are highly fluorescent. This is particularly true for red, orange, and yellow dyes in the azine (e.g., Safranin O), acridine (e.g., Acridine Orange), and xanthene (e.g., Rhodamine) families. Thus, many classical histological techniques unintentionally produce specimens that are intensely fluorescent. In addition, aldehyde fixation, certain mountants, and long-term storage of stained preparations can result in tissue fluorescence, and the high concentration of stains deposited in the sections can lead to metachromasia. In our hands, it is rare to find stained and sectioned botanical material that is not highly fluorescent.
The digital controls of a confocal microscope allow for the clean separation of different fluorescent emission signals and the balancing of signal levels in different channels. Thus, fluorescent images of exceptional clarity and vivid color can be easily obtained. In addition, the optical sectioning properties of the confocal microscope can be used to collect clear images from within thick sections and wholemounts.


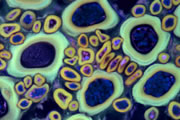
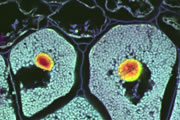
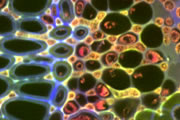

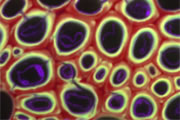
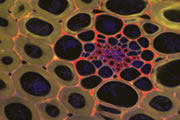
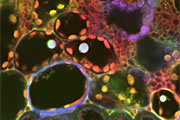
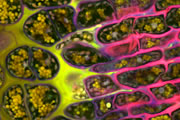
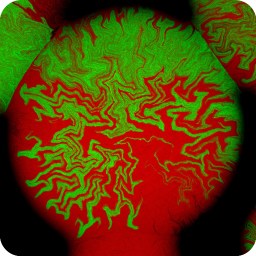
Different coloured fluorescent proteins are used routinely to decorate cells and subcellular structures in living tissues, and optical sectioning techniques allow the precise visualisation of these labels.The techniques are being used to map gene expression and visualise signalling events within the 3D cellular architecture of developing organisms.
Click here to see images of fluorescent protein expression in plants.
Click here to see images of fluorescent protein expression in plants.
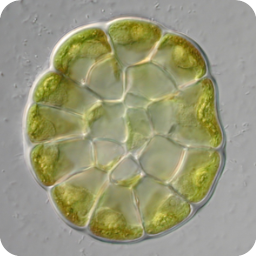
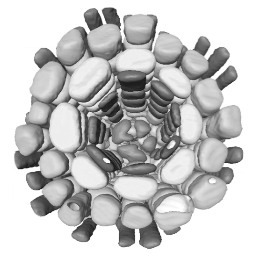
3D reconstruction of cell geometry and arrangements within intact plant tissues. The use of classical staining and clearing techniques with modern, optical sectioning confocal microscopes allows the 3D visualisation of plant tissues.
Plant Visions: 100 years of plant imaging in Cambridge. An exhibition curated by Beverley Glover and Jim Haseloff. Click to see images or download brochure.