High throughput - multiplex - single tube - isothermal - seamless - DNA assembly for everybody!
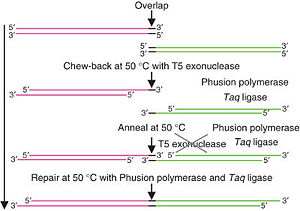
Gibson Assembly is a high-efficiency DNA end-linking technique developed by Daniel Gibson at the JCVI in 2009 [Gibson et al. 2009 plus supplementary methods]. The technique was invented and perfected as part of the genome assembly efforts at JCVI. The method uses three enzymes to join two or more sequences of DNA when they have overlapping end sequences at their joining point (~40bp). These overlapping regions can be easily added to the ends of any length of DNA by using PCR with primers which have added adapter sequences. Thus PCR followed by Gibson Assembly allows you to join any two blunt ended pieces of DNA. Up to 10-20 different pieces of DNA can be neatly spliced together in one reaction using these techniques.
Gibson Assembly is a high-efficiency DNA end-linking technique developed by Daniel Gibson at the JCVI in 2009 [Gibson et al. 2009 plus supplementary methods]. The technique was invented and perfected as part of the genome assembly efforts at JCVI. The method uses three enzymes to join two or more sequences of DNA when they have overlapping end sequences at their joining point (~40bp). These overlapping regions can be easily added to the ends of any length of DNA by using PCR with primers which have added adapter sequences. Thus PCR followed by Gibson Assembly allows you to join any two blunt ended pieces of DNA. Up to 10-20 different pieces of DNA can be neatly spliced together in one reaction using these techniques.
Advantages
1) No scar created - useful for fusion proteins and adding an RBS, where scars can be problematic.
2) Can re-ligate linear DNA into a circle - useful for site-directed mutagenesis
3) Any two blunt ended pieces of DNA can be joined, and DNA can be taken from any source which is accessible to PCR (for example, an organism's genome)
4) Although it is roughly the same speed as standard BioBrick assembly when ligating two fragments, Gibson Assembly comes into its own when assembling multi part systems, since it takes the same amount of time to ligate n pieces of DNA together as it does for two pieces.
Disadvantages
1) Primers must be ordered in advance
2) Additional cost of custom primers
In our hands at the University of Cambridge, the method has proved robust and efficient, and has effectively replaced restriction enzyme- DNA ligase style cloning, including standard BioBrick assemblies.
1) No scar created - useful for fusion proteins and adding an RBS, where scars can be problematic.
2) Can re-ligate linear DNA into a circle - useful for site-directed mutagenesis
3) Any two blunt ended pieces of DNA can be joined, and DNA can be taken from any source which is accessible to PCR (for example, an organism's genome)
4) Although it is roughly the same speed as standard BioBrick assembly when ligating two fragments, Gibson Assembly comes into its own when assembling multi part systems, since it takes the same amount of time to ligate n pieces of DNA together as it does for two pieces.
Disadvantages
1) Primers must be ordered in advance
2) Additional cost of custom primers
In our hands at the University of Cambridge, the method has proved robust and efficient, and has effectively replaced restriction enzyme- DNA ligase style cloning, including standard BioBrick assemblies.

https://youtu.be/WCWjJFU1be8
Video guide to Gibson Assembly by the University of Cambridge iGEM2010 Team.
Step One: Design and Order Primers

Step Two: PCR Amplify DNA parts
Step Three: Purify DNA fragments
Step Four: In vitro assembly
Step Five: Transformation and screening
These pages provide an open guide to the technique. We think that the method is of potential use to anyone engaged in DNA assembly and cloning. This is an ongoing effort - we are keen to explore ways of standardising DNA assembly and making biological engineering more efficient. In particular, we are keen to explore low cost education solutions for robotics, reactants and instrumentation.
Contributors:
Dr. Jim Haseloff, University of Cambridge (www.haseloff-lab.org and www.synbiostandards.co.uk)
Dr. Dean Madden, National Centre for Biotechnology Education (www.ncbe.reading.ac.uk)
Dr. Duncan Rowe, SABIC, KAUST, Saudi Arabia
Dr. Fernan Federici, PJ Steiner and James Brown, University of Cambridge.
Dr. Jim Ajioka, University of Cambridge (www.path.cam.ac.uk/research/investigators/ajioka/)
Dr. Emma Frow and Dr. Alistair Elfick, University of Edinburgh (www.synbiostandards.co.uk)
Contributors:
Dr. Jim Haseloff, University of Cambridge (www.haseloff-lab.org and www.synbiostandards.co.uk)
Dr. Dean Madden, National Centre for Biotechnology Education (www.ncbe.reading.ac.uk)
Dr. Duncan Rowe, SABIC, KAUST, Saudi Arabia
Dr. Fernan Federici, PJ Steiner and James Brown, University of Cambridge.
Dr. Jim Ajioka, University of Cambridge (www.path.cam.ac.uk/research/investigators/ajioka/)
Dr. Emma Frow and Dr. Alistair Elfick, University of Edinburgh (www.synbiostandards.co.uk)
...and especially - Bill Collins, Haydn King and the members of the University of Cambridge iGEM2010 and iGEM2011 Teams.
Bill Collins contributed the development of a series of web-based tools for DNA assembly, including Gibthon - a software tool for automated design of the oligonucleotides required for Gibson Assembly. Gibthon, instruction video and background information can be found at: www.gibthon.org
Bill Collins contributed the development of a series of web-based tools for DNA assembly, including Gibthon - a software tool for automated design of the oligonucleotides required for Gibson Assembly. Gibthon, instruction video and background information can be found at: www.gibthon.org