Chemical patterning - Belousov-Zhabatinsky reactions.
Week One - AM
You will be provided with aliquots of the components of the BZ reaction:
Acidified sodium bromate (0.5M NaBrO3, 0.5M H2SO4), acidified malonic acid (0.4M CH2(COOH)2, 0.5M H2SO4, plus 0.1% (w/v) SDS detergent, and a solution of ferroin, a redox indicator (25mM Fe (1,10-phenanthroline)3 SO4)
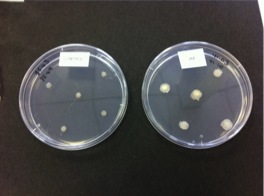
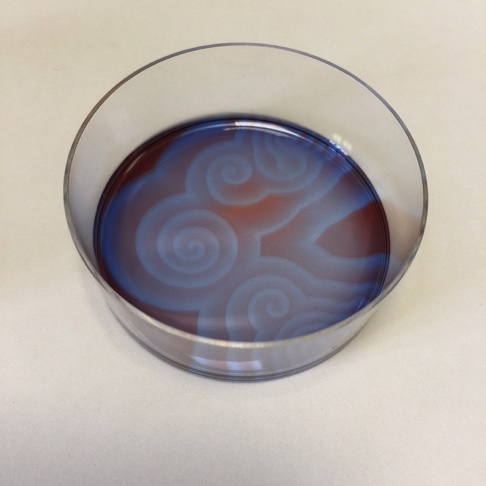
1. Mix the reactants Combine the reactants in a 5 cm diameter Petri dish. Be sure to wear gloves and use protective eye wear. Gently swirl the plate to ensure mixing of the reactants. Ferroin is a bright red colour. After mixing the solution should immediately turn a bright blue colour, followed by darkened state.
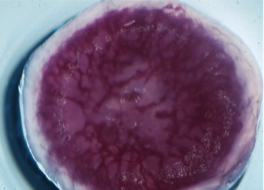
2. Spontaneous formation of oscillatory waves The solution should be left undisturbed, preferably on a white coloured surface. The solution will eventually turn again to a red colour. If the solution is held in a thin layer, points within the solution will be seen to form in blue tinted relief from the rest of the red solution. If left to develop undisturbed, these points will form travelling waves, where the reaction behaves as an excitable medium. Patterns should develop spontaneously over the course of and hour or two - even reforming if the solution is disturbed.
You will be provided with aliquots of the components of the BZ reaction:
Acidified sodium bromate (0.5M NaBrO3, 0.5M H2SO4), acidified malonic acid (0.4M CH2(COOH)2, 0.5M H2SO4, plus 0.1% (w/v) SDS detergent, and a solution of ferroin, a redox indicator (25mM Fe (1,10-phenanthroline)3 SO4)
1. Mix the reactants Combine the reactants in a 5 cm diameter Petri dish. Be sure to wear gloves and use protective eye wear. Gently swirl the plate to ensure mixing of the reactants. Ferroin is a bright red colour. After mixing the solution should immediately turn a bright blue colour, followed by darkened state.
2. Spontaneous formation of oscillatory waves The solution should be left undisturbed, preferably on a white coloured surface. The solution will eventually turn again to a red colour. If the solution is held in a thin layer, points within the solution will be seen to form in blue tinted relief from the rest of the red solution. If left to develop undisturbed, these points will form travelling waves, where the reaction behaves as an excitable medium. Patterns should develop spontaneously over the course of and hour or two - even reforming if the solution is disturbed.
In vitro patterning and differentiation of xylem elements in Jerusalem artichoke explants.
Week One - PM
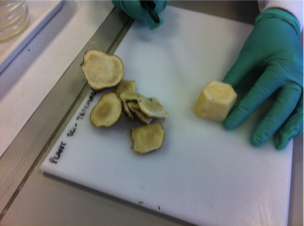
1. Trim Jeruslem artichoke tuber
2. Surface sterilise with sodium hypochlorite solution
3. Transfer under sterile “tent”
4. Wash 3x with sterile water
5. Trim top and bottom of tuber piece with large scalpel on sterile petri dish lid
6. Take cores of pith
7. Slice cores into 1mm explants & transfer to small beaker of sterile water
8. Wash twice with sterile water
9. Transfer to plates: weigh plate immediately before and after addition of explants to obtain fresh weight.
10. Wrap in aluminium foil - incubate for 1 week at 25C
1. Trim Jeruslem artichoke tuber
2. Surface sterilise with sodium hypochlorite solution
3. Transfer under sterile “tent”
4. Wash 3x with sterile water
5. Trim top and bottom of tuber piece with large scalpel on sterile petri dish lid
6. Take cores of pith
7. Slice cores into 1mm explants & transfer to small beaker of sterile water
8. Wash twice with sterile water
9. Transfer to plates: weigh plate immediately before and after addition of explants to obtain fresh weight.
10. Wrap in aluminium foil - incubate for 1 week at 25C


Week Two
1. Inspect tissue discs and note any differences
2. Weigh the tissue discs in plastic weigh boats
3. Incubate in 5% NaOH solution at 80C for 1 hour
4. Wash several times with water
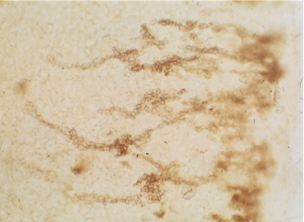
5. Incubate in 0.03% aqueous safranin O for 0.5 hour at 55C
6. Destain in several changes of 1M HCl over the period of an hour
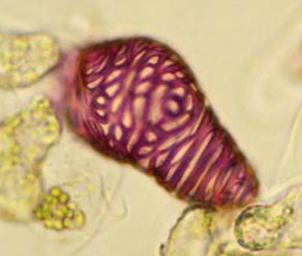
7. Inspect pattern of differentiated cells within intact discs
8. Transfer discs to small beakers and sonicate
9. Count differentiated cells in haemocytometer slide
10. Calculate numbers of differentiated cells (per g fresh weight) for the different treatments
1. Inspect tissue discs and note any differences
2. Weigh the tissue discs in plastic weigh boats
3. Incubate in 5% NaOH solution at 80C for 1 hour
4. Wash several times with water
5. Incubate in 0.03% aqueous safranin O for 0.5 hour at 55C
6. Destain in several changes of 1M HCl over the period of an hour
7. Inspect pattern of differentiated cells within intact discs
8. Transfer discs to small beakers and sonicate
9. Count differentiated cells in haemocytometer slide
10. Calculate numbers of differentiated cells (per g fresh weight) for the different treatments