
We are using primitive plant systems, members of the genus Coleochaete, as a testbed for studying the process of morphogenesis in plants. This genus of freshwater algae includes species with filamentous, branching and pseudoparenchymatous cell structure. Phylogenetic studies suggest that these microscopic algae are related to the direct antecedants of land plants. In particular, Coleochaete orbicularis can grow as a flat disc, comprising a single layer of cells on a flat substrate. It is a perfect subject for advanced microscopy techniques, and can be manipulated like a microorganism. Our aim is to combine the unique experimental benefits of the organism with a comprehensive computer model for the physical and genetic interactions that drive morphogenesis. This will allow direct visualisation and analysis of physical and genetic parameters in vivo and in silico, and testing of alternate morphogenetic models.
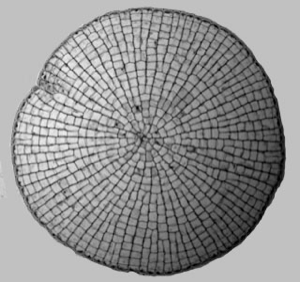
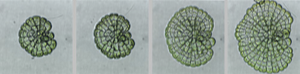
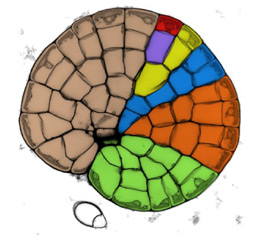
The meristematic zone is limited to a single layer of cells on the circumference of the growing disc. In evolutionary terms, these algae are poised between simpler unicellular and filamentous algae and the first plants, and show the first forms of parenchymatous growth. Coleochaetales are found in the fossil record, dating back over 400 Ma, to around the time of origin of the first terrestrial plants. Phylogenetic analysis of DNA sequences has identified C. orbicularis as a close living relative of land plants, and it possesses evolutionary novelties that are found in modern plants. Cell division occurs via phragmoplasts, rather than the phycoplasts found in lower algae, and produces parenchymatous tissues. Cell walls are cellulosic, and contain plasmodesmata. Zygotes are retained on the thallus during sexual reproduction, and are surrounded by specialised vegetative cells. Resistant spores are formed that contain sporopollenin, and colonies have a cuticle-like exterior that provides resistance to dessication. Colonies will grow freely in a moist terrestrial environment, and it is widely held that ancestral relatives may have made the transition to land.

Algal Culture. The algae can be grown as a suspension in shaken flasks, immobilised on culture dishes like animal cells or on the surface of agar plates like microbes. The colonies are robust and easy to culture, requiring only a minimal medium and light source.
Clonal Propagation. Coleochaete is haploid and reproduces asexually. Cells within the body of a thallus redifferentiate as motile zoospores with two flagella, form a pore in the upper surface of the cell and swim away from the parent colony. If allowed to settle, the zoospores will lodge on the substrate and commence cell division to produce a new colony. In this way, cultures can be rapidly amplified as clones.
Single Cell Isolation. Protoplasts can be harvested from Coloechaete colonies by digestion with cellulases and pectinases. This allows a ready supply of single cells for mutagenesis or transformation studies. Isolated protoplasts show very high capacity for regeneration. Washed protoplasts can be seeded onto solid agar media or a flat culture dish and they will form algal colonies readily.
High Resolution Microscopy. Zoospores or protoplasts can be used to inoculate a plastic culture dish or coverslip based vessel. With pretreatment of the surfaces, the cells will stick avidly. The subsequent growth of colonies can be followed by microscopy with great clarity. Full cellular detail can be seen even with low cost wide field microscopy, because the immobilised colonies grow as monolayers.
High Throughput Imaging. In collaboration with Leica Cambridge UK, we have developed techniques for high throughput time lapse microscopy of live colonies, using a computer controlled inverted microscope with a robotic stage. Large areas can be scanned and many fields of view can be defined in software, and sampled repetitively to build up parallel collections of timelapse data. The microscope contains DIC and fluoresence optics with a sensitive digital camera for the acquisition of multiparameter images. This automated approach shows much promise for use as an analytical tool and for high throughput screening.